Bioanalytical assays, notably ELISA assays, are crucial in drug discovery and development. These assays are indispensable tools for assessing drug efficacy and safety profiles. Adhering meticulously to the guidelines outlined in bioanalytical method validation (BMV), one must conduct thorough assay validation, encompassing ELISA immunoassays, across various stages such as preclinical safety evaluations, toxicokinetic assessments, and clinical trials.
ELISA analysis stands as an immunoassay employed extensively in measuring antibodies, antigens, proteins, and glycoproteins within biological samples.
In this blog, we will discuss the purpose, protocol, and result analysis of ELISA immunoassays.
The overarching purpose of ELISA assay development encompasses several critical applications, including:
- Detection of unknown antigens and antibodies in a given sample.
- Measurement of tumor marker levels for diagnostic and prognostic purposes.
- Quantification of hormone levels to assess endocrine function.
- Screening donated blood for potential viral contaminants, ensuring blood safety.
- Identification of drug abuse by detecting specific substances in biological fluids.
- Assessment of food allergens in the food industry, ensuring product safety.
- Determination of antibody concentration in serum for monitoring viral diseases.
- Surveillance of disease spread during endemic outbreaks, aiding public health interventions.
The protocols for conducting ELISA assays vary depending on the specific type of ELISA analysis being performed. Below is a detailed outline of the general protocol for conducting an ELISA:
Coating Antigen to the Microplate:
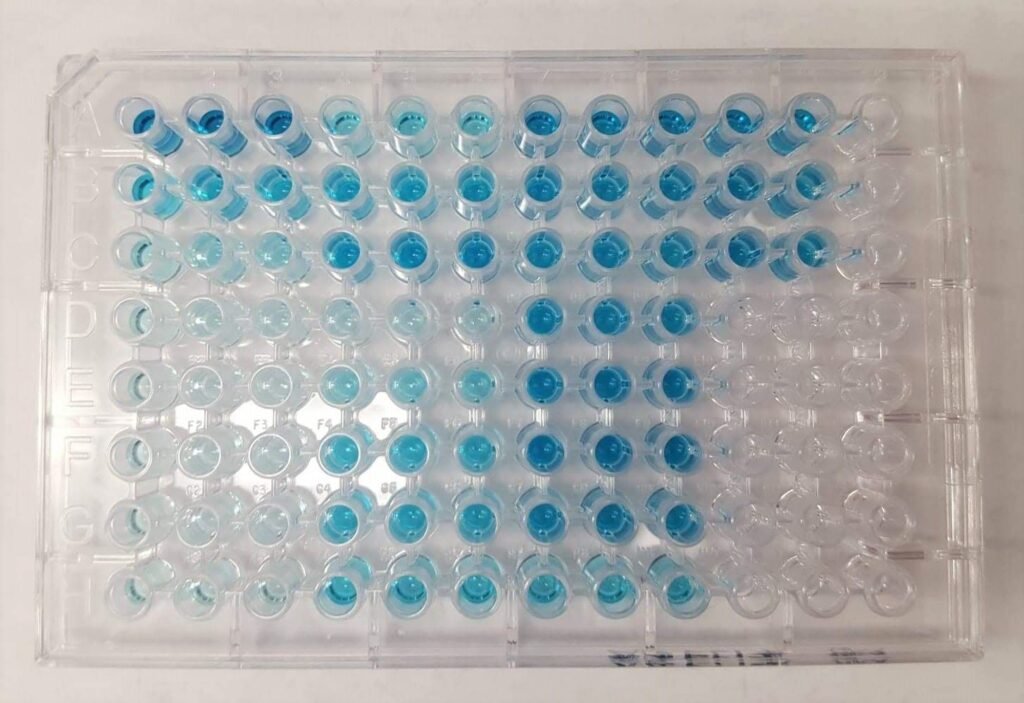
Commence the ELISA procedure by coating a 96-well ELISA plate with purified antigens, ensuring comprehensive coverage. Subsequently, cover the plate and incubate overnight to facilitate optimal antigen binding. Once the overnight incubation concludes, delicately remove any excess coating solution, ensuring thorough drainage before proceeding with subsequent steps in the assay.
Blocking:
Add a blocking buffer to the coated wells to prevent any residual protein-binding sites from interacting undesirably. Incubate the plate overnight at 4°C to allow for effective blocking. Following the incubation period, remove the blocking buffer and subsequently wash it with PBS containing 1% Tween-20 to ensure thorough removal of any unbound substances.
Must Read: The Role of PK Clinical Trials in Drug Development
Incubation with Antibodies:
Dilute the serum sample containing the primary antibody to achieve a dilution in the desired range. Subsequently, add the serially diluted serum samples to the wells of the ELISA plate. Cover the plate and incubate it at room temperature (RT). After the designated incubation period, remove any excess solution, followed by washing it with PBS containing 1% Tween-20 to ensure thorough removal of unbound substances. Proceed to incubate the plate with the secondary antibody at RT. Upon completion of the secondary antibody incubation, remove the excess solution and repeat the washing step with PBS containing 1% Tween-20 to prepare the plate for subsequent analysis.
Detection:
Add an indicator substrate to each well, ensuring uniform coverage. Incubate the plate at RT with the substrate for 5-10 minutes. After 10 minutes, halt the enzymatic reaction by carefully adding 2N Sulfuric acid. Within 30 minutes following the addition of the stop solution, utilize a microplate reader to read the plate, enabling the determination of the absorbance of the wells.
Result analysis:
There are three types of data outputs:
Quantitative:
It involves a comparison of the obtained results with a standard curve. This standard curve is constructed using serial dilutions of a known, purified antigen. Through this comparison, precise calculations of antigen concentrations in different samples can be achieved.
Qualitative:
ELISAs also can deliver a binary outcome, discerning the presence or absence of a specific antigen within a sample. This determination is made by comparing the signal obtained from the sample to that of a blank well. If the signal in the sample well exceeds a predetermined threshold, it suggests the presence of the target antigen; otherwise, it indicates its absence.
Semi-quantitative:
ELISAs enable the comparison of relative antigen levels in assay samples, attributed to the direct correlation between the intensity of the generated signal and the antigen concentration.
Today, laboratories offer specialized services like ELISA assay development service and other bioanalytical assay services, such as immunoassay services, only after a comprehensive understanding of study requirements. This approach ensures the prompt delivery of high-quality results tailored to individual needs. They stand ready to provide exceptional support throughout the process, maintaining seamless communication to meet the project’s critical deadlines. Additionally, assay lab operations are geared towards ensuring dependable biological assay validation, thereby guaranteeing the accuracy and reliability of research findings.




Hey there! Do you know if they make any plugins to assist with
Search Engine Optimization? I’m trying to get my website to
rank for some targeted keywords but I’m not seeing very good success.
If you know of any please share. Kudos! I saw similar text
here: Warm blankets
Hi there! Do you know if they make any plugins to help with Search Engine Optimization? I’m trying to get my website to rank for some targeted keywords but I’m not seeing very good success.
If you know of any please share. Appreciate it! I saw
similar article here: Your destiny